Wastewater Recovery RO Plant
Water Treatment Technologies
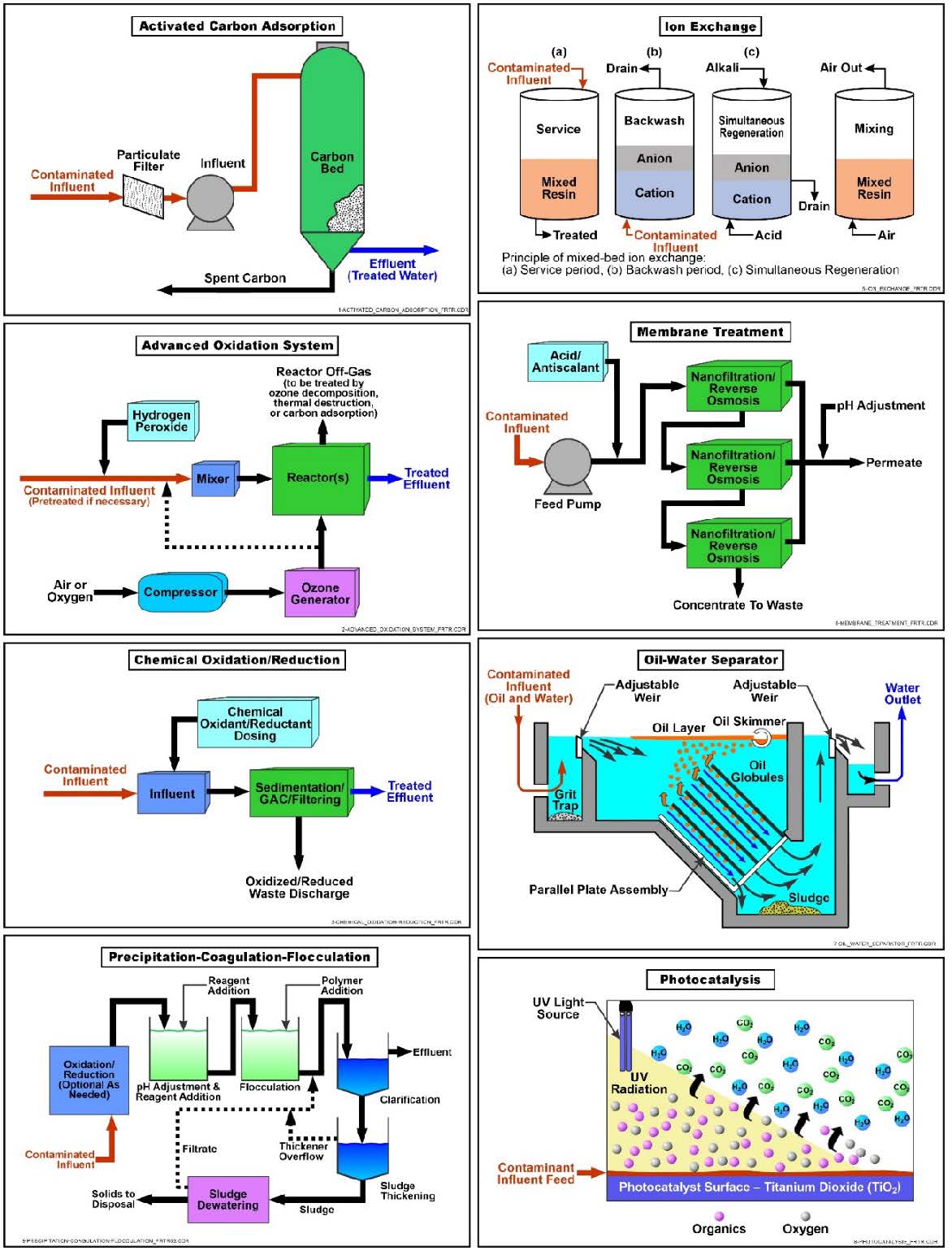
Schematic
This information may be reproduced without restriction as long as the source attribution is included.
Schematic of Common Water Treatment Technologies
Introduction
Many remediation technologies generate an aqueous stream that may require treatment depending on contaminant type and concentration, site location, and regulatory requirements. A variety of treatment options based on physical (e.g., oil water separation, adsorption, and filtration), chemical (oxidation, ion exchange, photocatalysis), and biological (e.g., biological filtration) processes are available to treat the water generated by these remediation technologies. These treatment technologies can be applied independently or as part of a treatment train to overcome site-specific limitations and remove a wider range of contaminants of concern (COCs) of different phases (non-aqueous phase liquid [NAPL], suspended solids, and dissolved) than possible using a single treatment technology. Each type of treatment has its advantages and limitations and may or may not be applicable to all COCs at a site. This profile describes commonly used contaminant treatment technologies, presents their basic principles and applicability, and describes their limitations and other considerations for implementation. In addition, beneficial reuse or reclamation of the treated water is described, which has gained more recognition in recent years as part of a sustainable remediation management strategy.
Other Technology Names
Pump and Treat
Dissolved Phase Treatment
Aqueous Phase Treatment
Description
Water treatment is required for many remediation technologies that remove contaminated groundwater from the aquifer during restoration activities. Water treatment usually consists of a treatment train to remove contaminants (e.g., oil globules, hydrocarbon constituents, chlorinated solvents, metals, and inorganic ions) and return the water to a condition that can be discharged/reused. The water treatment system must be designed based on the COCs present, their concentrations, and local regulatory requirements. Systems can be portable and temporary (on-site trailer mounted) or can be large and complex for long-term projects (e.g., pump and treat systems) housed in buildings constructed for this purpose. Water treatment is accomplished using a variety of technologies based on treatment processes commonly used by municipal and industrial wastewater treatment facilities. Common classes of treatment include:
Absorption
Adsorption consists of solutes concentrating at the surface of a sorbent, thereby reducing their concentration in the bulk liquid phase. Adsorption mechanisms are generally categorized as either physical adsorption, chemisorption, or electrostatic adsorption. Weak molecular forces, such as Van der Waal forces, provide the driving force for physical adsorption, while a chemical reaction forms a much stronger chemical bond between the compound and the surface of the solid in chemisorption. Electrostatic adsorption involves the adsorption of ions through Coulombic forces, and is normally referred to as ion exchange. For liquids, interactions between the solute and the solvent play an important role in establishing the degree of adsorption.
Commonly used natural and synthetic adsorbents are described below.
- Granular activated carbon (GAC) is the most common type of adsorbent used to treat water generated at environmental restoration sites. The carbon is “activated” by being processed to create porous particles having a large internal surface area (300 to 2,500 square meters or about 3,200 to 27,000 square feet per gram of carbon) that attracts and adsorbs organic molecules as well as certain metal and inorganic molecules.
- Activated alumina is a filter media made by treating aluminum ore so that it becomes porous and highly adsorptive. Activated alumina will remove a variety of contaminants, including excessive fluoride, arsenic, and selenium.
- Forage sponge is an open-celled cellulose sponge incorporating an amine-containing chelating polymer that selectively absorbs dissolved heavy metals. The polymer is bonded to the cellulose to minimize physical separation from the supporting matrix. The functional groups in the polymer (i.e., amine and carboxyl groups) provide selective affinity for heavy metals in both cationic and anionic states, preferentially forming complexes with transition-group heavy metals.
- Lignin clays are used to treat aqueous waste streams with organic, inorganic and heavy metals contamination. The waste stream is treated by the molecular adhesion of the contaminants to an adsorptive surface.
- Synthetic resins can achieve higher degrees of selectivity and adsorption capacity for certain compounds than activated carbon. Resins are typically regenerated using acids, bases, or organic solvents, instead of thermal methods, so they are better suited for thermally unstable compounds such as explosives. They are resistant to deactivation due to the adsorption of dissolved solids.
Adsorbents are placed in one or more vessels and groundwater is pumped through them. The two most common configurations for adsorption systems are the fixed bed and the pulsed or moving bed. The fixed-bed configuration is the most widely used for adsorption of contaminants from liquids.
Pretreatment for removal of suspended solids from streams to be treated is an important design consideration. If not removed, suspended solids in a liquid stream may accumulate in the bed, causing an increase in pressure drop. When the pressure drop becomes too high, the accumulated solids must be removed by backwashing. The solids removal process necessitates downtime and may result in carbon loss and disruption of the mass transfer zone.
When the concentration of contaminants in the effluent from the vessels exceeds a target level, depending on the adsorbent used and the types of contamination present, it may be possible to regenerate the adsorbent, usually at an off-site facility. However, in some cases, it is not possible to regenerate the material. For example, GAC used for explosives- or metals-contaminated groundwater probably cannot be regenerated and should be removed and properly disposed.
Air Stripping
Air stripping is an ex situ technology that removes volatile organic compounds (VOCs) from pumped groundwater or wastewater by passing the water over a media having a large surface area while exposing the contaminated water to uncontaminated air flow. The VOCs are transferred (i.e., volatilized) from the groundwater or wastewater to the vapor phase in the countercurrent air stream, where the vapor is either directly discharged or routed to an off-gas treatment system. Additional air stripping design and application information can be found here.
Chemical Oxidation and Reduction
Chemical oxidation refers to the conversion of hazardous contaminants using oxidants to non-hazardous or less toxic compounds. Commonly used oxidizing agents are ozone, hydrogen peroxide, permanganate, hypochlorites, chlorine, and chlorine dioxide. These oxidants can degrade many toxic organic chemicals, control biological growth in treatment units, and convert metals (such as iron and manganese) into forms that are more amenable to precipitation and other physical separation processes. Oxidants are able to achieve high treatment efficiencies with very fast reaction rates (e.g., > 90 percent in minutes) for unsaturated aliphatic (e.g., trichloroethylene [TCE]) and aromatic compounds (e.g., benzene).
Commonly used oxidants include:
- Fenton’s reagent, which consists of hydrogen peroxide in the presence of native or supplemental ferrous iron that catalytically reacts to form free hydroxyl radicals. These strong, nonspecific oxidants can rapidly degrade a variety of organic compounds. Fenton’s reagent oxidation is most effective under very acidic pH (e.g., pH 2 to 4)
and becomes ineffective under moderate to strongly alkaline conditions.
- Ozone gas, which can oxidize contaminants directly or through the formation of hydroxyl radicals. Like peroxide, ozone reactions are most effective in systems with acidic pH. The oxidation reaction process is extremely fast. Ozone must be generated on site and used immediately due to its short half-life, typically less than 30 minutes.
- Permanganate, which generally is applied in either sodium or potassium (permanganate) form. The reaction stoichiometry of permanganate in natural systems is complex. The reactions proceed at a somewhat slower rate than those of the two abovementioned oxidants. Permanganate reactions are effective over a pH range of 3.5 to 12.
- Chlorine, which can be applied as a liquid (e.g., sodium hypochlorite) or as a gas (chlorine gas), to control biological growth and to remove color, taste and odor compounds, iron and other dissolved inorganic contaminants such as arsenic. It should be noted, however, that chlorine reacts with natural organic compounds in water to form potentially chemical byproducts including trihalomethanes and haloacetic acids, although this may not be a significant concern for most applications for treating recovered groundwater.
Chemical reduction is the opposite of chemical oxidation. It involves reducing oxidized species of contaminants (or residual oxidants). Common reductants include ferrous sulfate and sodium sulfite. Chemical reduction is typically used in water treatment to remove oxygen, reduce hexavalent chromium, and destroy residual oxidants. In reducing oxygen, sodium sulfite or ammonium bisulfite which, although more expensive, is easier to use and has a much more extensive buffer effect. The chemical removal of total chromium involves reducing hexavalent chromium to trivalent chromium followed by precipitation as a hydroxide. The destruction of residual oxidants typically occurs after wastewater disinfection in order to limit the subsequent formation of unwanted byproducts, before processing the water through a membrane or ion exchanger resin, or before discharging or recycling gas from ozone utilization applications.
Advanced Oxidation Processes (AOPs)
AOPs refer to the destruction of organic contaminants using a form of light radiation in conjunction with oxidants (e.g., ozone or hydrogen peroxide) or a catalyst to achieve rapid and efficient destruction of COCs. AOPs can be divided into two primary types of processes including:
- Ultraviolet (UV) oxidation, which is a destruction process that can oxidize organic and explosive constituents in water by the addition of strong oxidizers such as ozone and/or hydrogen peroxide, and irradiation with UV light. Oxidation of target contaminants is caused by direct reaction with the oxidizers, UV photolysis, and through the synergistic action of UV light.
If complete degradation is achieved, the final products of UV oxidation are carbon dioxide, water, and salts. Systems can be configured in batch or continuous flow modes, depending on the throughput under consideration. The UV oxidation process is generally performed using low pressure lamps operated at 65 watts for ozone systems and 15 to 60 kilowatts for hydrogen peroxide systems. The duration of operation and maintenance of UV oxidation depends on influent water turbidity, contaminant and metal concentrations, existence of free radical scavengers, and the required maintenance intervals on UV reactors and quartz sleeves.
- Photocatalysis uses visible light and a catalyst to treat a range of organic contaminants. Light is absorbed by a substrate and the photocatalytic activity depends on the ability of the catalyst to generate free radicals (e.g., hydroxyl radicals) able to undergo secondary reactions. Photocatalysis is induced under UV, solar, and visible light and can be classified as homogeneous and heterogeneous. Photolysis is a weaker process than UV oxidation. Products of photo-degradation vary according to the matrix in which the process occurs, but the complete conversion of an organic contaminant to carbon dioxide, water, etc. is not probable.
Filtration (Particulate)
Filtration is the physical process of mechanical separation based on particle size whereby particles suspended in a fluid are separated by forcing the fluid through a porous medium. As fluid passes through the medium, the suspended particles are trapped on the surface of the medium and/or within its body. Common types of filtration techniques include:
- Sand filtration, which consists of a filter box filled with a bed of fine sand supported by a layer of gravel, and particulate and microbial contaminants are removed when water percolates through the filter box. It is used when water contains relatively low levels of suspended contaminants. It may involve the addition of a coagulant followed by rapid mixing, flocculation and filtration.
- Biological filtration, in which contaminants are removed by three main mechanisms: biodegradation, adsorption of micropollutants, and filtration of suspended solids. The filter usually consists of a bed comprised of sand or crushed gravel which hosts a community of microorganisms that degrade the contaminants as the water is passed through the filter.
- Membrane filtration occurs when particles are separated by forcing fluid through a semipermeable membrane. Only the particles whose sizes are smaller than the openings of the membrane can flow through it. Microfiltration (MF) and ultrafiltration (UF) are most commonly used in water treatment and can be operated under positive or negative pressure (i.e., vacuum pressure). Membrane pore size for MF units typically ranges between 0.1 to 0.5 micrometer (µm) while UF pore sizes range from about 0.01 to 0.1 µm. Particulate contaminants are retained by the membrane and removed during periodic backwashing of the membrane filter. Flux rates for MF and UF membranes typically range between 40 and 100 gallons per day per square foot of membrane area. The design flux is typically determined by bench or pilot testing since it is dependent on specific water quality conditions and temperatures.
Membrane Separation (Dissolved)
Membrane filtration also may be used to remove dissolved contaminants. Membrane separation processes for this purpose include reverse osmosis (RO), nanofiltration (NF), electrodialysis (ED), and electrodialysis reversal (EDR):
- RO and NF both operate based on the principle of reverse osmosis. Osmosis is the natural flow of a solvent, such as water, through a semi-permeable membrane (which acts as a barrier to dissolved contaminants) from a less concentrated solution to a more concentrated solution. Flow continues until the concentrations on each side of the membrane are equal. The amount of pressure that must be applied to stop this flow of water is called the osmotic pressure. RO is the reversal of the natural osmotic process by applying pressure in excess of the osmotic pressure to the more concentrated solution. The feed pressure forces the water through the membrane against the natural osmotic gradient, thereby increasing the dissolved contaminant concentrations on one side of the membrane and increasing the volume of water with lower concentrations of dissolved contaminants on the other. The principal difference between RO and NF is the composition of the membrane, which affects the size of dissolved contaminants that can be removed. Nanofiltration membranes don’t remove as small molecular weight contaminants as RO but operate at a lower pressure than RO membranes and therefore consume less power than RO membranes. However, NF can provide very high removal of multivalent ions with low to moderate removal of monovalent ions, and selective removal of organic compounds determined by molecular weight, size, and charge relative to the molecular weight, pore size, and charge of the membrane material.
- ED is an electrochemical separation process in which ions are transferred through membranes from a less concentrated to a more concentrated solution as a result of the flow of direct electric current. ED systems consist of alternating anion and cation ion exchange membranes placed between positive and negative electrodes. Applying a voltage across the electrodes causes the positively charged cations to move towards the negative electrode and negatively charged anions to move towards the positive electrode. Contaminants are removed from the water through the membrane, as opposed to other membrane processes where the water passes through the membrane and the contaminants are rejected by the membranes.
- EDR is a process similar to ED, but the electrical polarity (anode and cathode) is periodically reversed to control membrane scaling and fouling. Polarity reversal typically occurs two to four times per hour. When the electrical polarity is reversed, the product and concentrate streams are also reversed. This prevents the flow compartments from seeing streams with high dissolved solids for extended periods of time and aids in controlling fouling.
These membrane separation processes include three basic flow streams: the feed, permeate or product, and concentrate or waste streams. For RO and NF, treatment is generally in stages, wherein the concentrate from the prior stage becomes the feed for the subsequent stage. The permeate from each stage is blended together for the final product stream. The concentrate from the final stage is usually wasted (disposed). In ER and EDR, the product from the prior stage is further treated in subsequent stages.
The concentration of other impurities (e.g., calcium carbonate, iron, organic matter, and bacteria) in the concentrate stream limits recovery by membrane systems. Temperature can significantly impact membrane performance. A decrease in temperature will result in the increase of viscosity and density, and the transmembrane pressure required to pass the water through the membrane also increases, resulting in an increase in the specific flux. Contaminant solubility also decreases as temperature decreases which may require operation at a lower recovery to prevent scaling. For ED and EDR systems, water temperature affects ion mobility. Higher temperatures can cause membrane degradation and compaction of RO and NF membranes.
Residuals generated from membrane separation systems include the concentrate from the membrane processes and spent cleaning chemicals. Chemical cleaning is required to periodically remove scale buildup and biological fouling on the membrane surface. Spent solutions are generally acidic in nature and require neutralization prior to disposal.
Ion Exchange
Ion exchange removes ions from the aqueous phase by the exchange of cations or anions between the contaminants and the exchange medium. It involves passing contaminated water through an ion exchange resin so that contaminants exchange on sites on the media, exhausting its capacity. After the resin capacity has been exhausted, resins can be regenerated for reuse. The resin material typically consists of synthetic organic polymers that contain ionic functional groups to which exchangeable ions are attached. Resins are categorized as strong or weak acid cation exchangers or strong or weak base anion exchangers. Cationic resins are typically used for softening (e.g., calcium and magnesium removal), while anionic resins are used to remove various anions impacting water quality (e.g., perchlorate and nitrate). Recent advances include application of contaminant-selective ion exchange resins that have high capacities for a single target contaminant and are disposed after use instead of regenerated. For example, perchlorate-selective resins and arsenic-selective resins are commercially available.
Metal Sequestration
Metal sequestration is a form of treatment in which a chemical, known as a sequestrant or sequestering agent, is added to water. Most sequestering chemicals are polyphosphate compounds (e.g., sodium- and potassium-based), which provide nutrients to microorganisms and may need to be treated prior to discharging the water. The chemical forms a bond with metal ions (e.g., iron and manganese) or heavy metals, allowing them to remain in solution and prevent further reactions (e.g., oxidation and precipitation) that might interfere with the treatment process (e.g., fouling of equipment) or cause water quality problems (turbidity, color, staining, etc.). The effectiveness of sequestering agents depends on influent water quality in addition to metal levels. Bench- or pilot-scale testing typically is conducted prior to application of sequestering agents. Sequestered metals can be filtered and removed more easily in a later process. If not removed, sequestered metals may be released back to the water stream, causing treatment or water quality problems in subsequent processes or discharge.
Oil Water Separation
Oil water separation is the process of separating oil (i.e., NAPL) from the water stream generated by other remediation technologies such as multi-phase extraction and free product recovery. Available methods for separating oil water mixtures include one or more physical, chemical, mechanical, electrical, magnetic, and thermal mechanisms; however, gravity differential separation is by far the most commonly used method to separate oil water mixtures generated at environmental restoration sites.
There are several types of gravity separators including American Petroleum Institute (API) oil/water separators, circular and plate interceptors, rotational separators (centrifuges, vortex flow), and gas flotation separators. Gravity separation is basic to almost all oil water separators. It is usually the first step in the treatment of oily waters and provides coarse separation of oil and water. In general, oil water mixtures will separate naturally into two distinct layers of oil and water. Oil globules and/or suspended particles will rise to the surface (e.g., light NAPL or LNAPL) or fall to the bottom (e.g., dense NAPL or DNAPL), depending on the sign of the density differential. In the case of LNAPL, oil globules rise and form a floating layer, which is easily skimmed from the surface. Ease of separation depends on the magnitude of the difference in densities of the two immiscible liquids, and size of the oil globules or droplets. Gravity separation is inefficient when the density difference is small and oil droplets are small. Devices operating on the gravity principle will not separate dissolved oil or emulsions.
pH Control
pH is an indicator of the acid or alkaline condition of water. The pH of a water stream is a result of natural geological and chemical conditions and any preceding remediation processes. pH control or adjustment is the alteration of pH by adding a reagent such as an acid to drive pH down or a caustic or other alkaline substance to increase pH. pH control or adjustment can be used to directly treat contaminants (e.g., metal precipitation and oil emulsion breaking) or create favorable conditions for other treatment processes (e.g., coagulation, chemical oxidation, and biological treatment). For example, the optimal pH range for coagulation is 6 to 7 when using aluminum sulfate (alum) and 5.5 to 6.5 when using iron, and the optimal pH range for treatment involving biological degradation is normally near neutral (6.5 to 7.5).
Sulfuric acid, hydrochloric acid, calcium/sodium carbonate, and sodium hydroxide are most commonly used for pH control. They can be added to the treatment process via tanks and mixers or dosed directly into the process line and mixed using static mixing elements controls, continuously or in batches.
Control of pH to near neutral can be achieved through a neutralizing filter. The filter contains a neutralizing material that dissolves in the influent water and raises its pH level. Calcium carbonate treats water with a pH greater than 6 and synthetic magnesium oxide will treat water with a pH below 6.
Precipitation/Coagulation/Flocculation
Precipitation, coagulation, and flocculation processes transform dissolved contaminants into an insoluble solid that is easily separated by sedimentation or filtration. Typically used to treat metals or facilitate removal of oils in contaminated water, this treatment process is often used as a pretreatment for other treatment technologies such as chemical oxidation where the presence of metals would interfere with these processes. Metals precipitation from contaminated water involves the conversion of soluble heavy metal salts to insoluble salts that will precipitate. The precipitate can then be removed from the treated water by physical methods such as settling and/or filtration. The process usually requires pH adjustment, addition of a chemical precipitant and a flocculant (e.g., polymer). Typically, metals precipitate from the solution as hydroxides, sulfides, or carbonates, while oils will adhere to the coagulant. Depending on the specific gravity of the resulting material, it can be removed as a sludge from the bottom of a separator or skimmed from the surface. The solubilities of the specific contaminants and the required cleanup standards will dictate the process used. In some cases, process design will allow for the generation of sludges that can be sent to recyclers for metal or oil recovery.
In the precipitation process, chemical precipitants, coagulants, and flocculants are used to increase particle size through aggregation. The precipitation process can generate very fine particles that are held in suspension by electrostatic surface charges. These charges cause clouds of counter-ions to form around the particles, giving rise to repulsive forces that prevent aggregation and reduce the effectiveness of subsequent solid-liquid separation processes. Therefore, chemical coagulants are often added to overcome the repulsive forces of the particles. The addition of coagulants is followed by low-sheer mixing in a flocculator to promote contact between the particles, allowing particle growth through the sedimentation phenomenon called flocculant settling. Bench-scale treatability tests should be conducted to determine operating parameters and characteristics (e.g., reagent type and dosage, optimum pH, retention time, flocculent/polymer selection, suspended solids, precipitate settling and filtration rates, and sludge volume and characteristics).
Beneficial Reuse/Reclamation
Beneficial reuse and reclamation of treated water is an option in lieu of disposal that contributes to the overall conservation of water, and has gained more recognition in recent years. Partially or fully treated water can be reinjected into a deeper aquifer, reinjected into a treatment area to improve capture and control plume migration, sprinkled onto the ground for irrigation or to remove residual contamination, or discharged to lagoons and percolation ponds to replenish aquifers.
Beneficial reuse and reclamation of the treated water has been advocated as part of the sustainable or green remediation concept. Sustainable remediation considers the environmental, social, and economic aspects of potential groundwater conservation and reuse options in remediation projects. Remediation activities that are costly and less efficient can become attractive when considering their positive contribution to the environment. For example, groundwater pump and treat historically is considered a less effective and efficient strategy for groundwater restoration projects because of the difficulty in removing contaminants from the aquifer matrix and the long duration of operation required. However, from a groundwater reuse perspective, groundwater pump-and-treat systems might be viewed differently when the demand for the treated water is high.
In the past several decades, there have been numerous successful beneficial reuse applications ranging from small-scale reuse (e.g., cooling water and dust control) to regional-scale reclamation (e.g., aquifer replenishment and saltwater intrusion prevention projects). One such example is at the former Unidynamics Phoenix, Inc. facility in central Arizona, which produces an aggregate flow of approximately 2,800 gpm from the extraction wells. Approximately 1,600 gpm of treated groundwater is returned to the aquifer via injection wells and the remainder 1,200 gpm is used for dust control, irrigation of local agriculture, a golf course, and a local city park, and precooling of HVAC cooling water by a local school. In another application, extracted groundwater containing chlorinated VOCs is pumped directly into a steel mill’s contact cooling water system, where the chlorinated VOCs are volatilized in the steel cooling process. Remediation sites within states having water reuse regulations or guidelines should make reuse or reclamation an option in evaluating remediation strategies.
These applications are subject to state-specific rules as water reuse regulation is generally under the jurisdiction of the states. The regulations developed by states vary, and some states have yet to develop water reuse guidelines or regulations.
















